Manufacturing Process: The Fundamental Idea
The fundamental idea of manufacturing or production is to create, (or produce), something that has a useful form. This form is most likely predetermined and calculated, with a certain physical geometry. Usually this geometry has certain tolerances that it must meet in order to be considered acceptable. A tolerance outlines the geometric accuracy that must be achieved in the manufacturing process. The "tightness" of the tolerances, or in other words the allowed variance between the manufactured product and the ideal product, is a function of the particular application of the product.
Manufacturing Materials
All manufactured products are made from some sort of material. Similar to the geometric tolerance, the properties of the material of the final manufactured product are of utmost importance. Hence, those who are interested in manufacturing should be very concerned with material selection. An extremely wide variety of materials are available to the manufacturer today. The manufacturer must consider the properties of these materials with respect to the desired properties of the manufactured goods. Simultaneously, one must also consider manufacturing process. Although the properties of a material may be great, it may not be able to effectively, or economically, be processed into a useful form. Also, since the microscopic structure of materials is often changed through different manufacturing processes -dependent upon the process- variations in manufacturing technique may yield different results in the end product. Therefore, a constant feedback must exist between manufacturing process and materials optimization.
Basic Concepts:
Types of Materials
Materials can be classified into 3 basic types.
1.Metals
2.Ceramics
3.Polymers
What are the properties of metals, the properties of ceramics, the properties of polymers?
Metals: Metals are hard, malleable, (meaning capable of being shaped), and somewhat flexible materials. Metals are also very strong. Their combination of strength and flexibility makes them useful in structural applications. When the surface of a metal is polished it has a lustrous appearance; although this surface luster is usually obscured by the presence of dirt, grease and salt. Metals are not transparent to visible light. Also, metals are extremely good conductors of electricity and heat.
Ceramics: Ceramics are very hard and strong, but lack flexibility making them brittle. Ceramics are extremely resistant to high temperatures and chemicals. Ceramics can typically withstand more brutal environments than metals or polymers. Ceramics are usually not good conductors of electricity or heat.
Polymers: Polymers are mostly soft and not as strong as metals or ceramics. Polymers can be extremely flexible. Low density and viscous behavior under elevated temperatures are typical polymer traits. Polymers can be insulative to electricity.
What are metals made of, what are ceramics made of, what are polymers made of?
Or in other words, what is the basic microstructure of metals, what is the basic microstructure of ceramics, what is the basic microstructure of polymers?
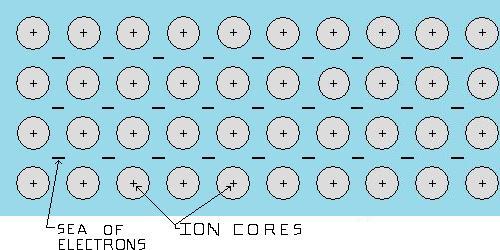
Metals: A metal is most likely a pure metallic element, (like iron), or an alloy, which is a combination of two or more metallic elements, (like copper-nickel), the atoms of a metal, similar to the atoms of a ceramic or polymer, are held together by electrical forces. The electrical bonding in metals is termed metallic bonding. The simplest explanation for these types of bonding forces would be positively charged ion cores of the element, (nucleus's of the atoms and all electrons not in the valence level), held together by a surrounding "sea" of electrons, (valence electrons from the atoms). With the electrons in the "sea" moving about, not bound to any particular atom. This is what gives metals their properties such malleability and high conductivity. Metal manufacturing processes usually begin in a casting foundry.
Figure 1
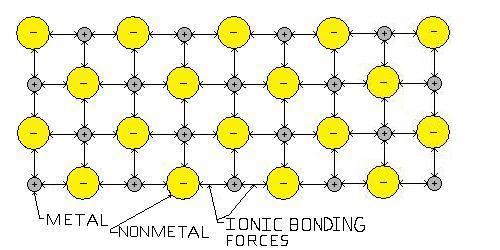
Ceramics: Ceramics are compounds between metallic and non-metallic elements. The atomic bonds are usually ionic, where one atom, (non-metal), holds the electrons from another, (metal). The non-metal is then negatively charged and the metal positively charged. The opposite charge causes them to bond together electrically. Sometimes the forces are partially covalent. Covalent bonding means the electrons are shared by both atoms, in this case electrical forces between the two atoms still result from the difference in charge, holding them together. To simplify think of a building framework structure. This is what gives ceramics their properties such as strength and low flexibility.
Figure 2
Polymers: Polymers are often composed of organic compounds and consist of long hydro-carbon chains. Chains of carbon, hydrogen and often other elements or compounds covalently bonded together.
Figure 3
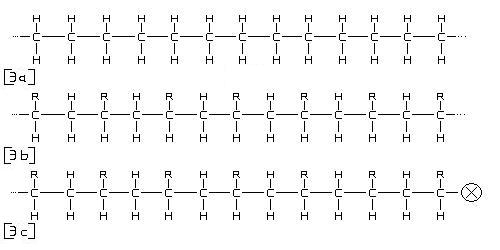
In figure 3, (a) represents a simple hydrocarbon chain, each group of hydrogen and carbon is called a mer, there are 13 mers shown in the diagram, the dotted lines indicate that the pattern is continuing indefinitely. Polymers chains often contain thousands upon thousands of mers each. The [R] in (b) indicates a variable element or group of elements that could occupy a certain position in the chain. The [X] in (c) also represents another variable element or group that could occupy another position, this one being at the end or beginning of a polymer chain. The chains themselves bond to each other through secondary bonding forces. To simplify polymer structure, think of a bowl of spaghetti.
Figure 4
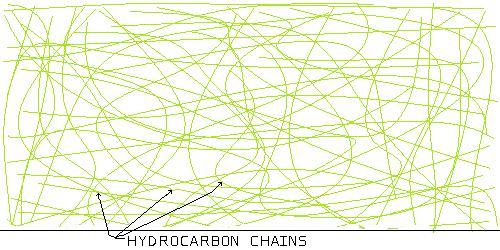
This is a polymer with a random or amorphous microstructure.
Figure 5
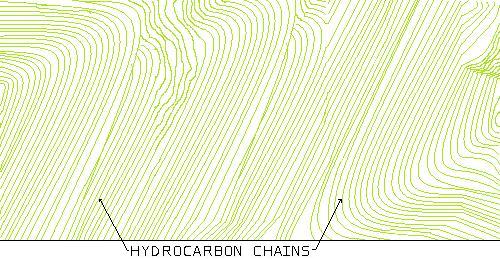
This is a polymer with a very high degree of order or a crystalline microstructure.
When heat is applied, the weaker secondary bonds, (between the strands), begin to break and the chains start to slide easier over one another. However, the stronger covalent bonds, (the strands themselves), stay intact until a much higher temperature. This is what causes polymers to become increasingly viscous as temperature goes up.
Copyright © Jiangsu Hoston Machine Tools Co., Ltd. (Hongkong Hoston Group CO., LIMITED)All Rights Reserved | Sitemap Technical Support: 